40+ How To Calculate Molar Solubility From Ksp
Web The more soluble a substance is the higher the Ksp value it has. Is Ksp the same as molar solubility.
Calculate Solubility Of Silver Benzoate In A Buffer Solution Of Ph 3 19 The Ionisation Cons An T Of Benzoic Acid Is 6 46 10 5 And Ksp For Silver Benzoate Is 2 5 10 13 B Also Calculate
The terms molar solubility and solubility product may be confusing as they are similar but not the sameThe solubility product is the K sp which is the product.

. Web To calculate the Molar Concentration we will find the molar concentration by dividing the moles by liters of water used in the solution. Web Molar solubilityis the number of moles of a substance the solute that can be dissolved per liter of solution before the solution becomes saturated. Web How to calculate the KSP of a compound.
It can be calculated. Web The solubility constant expression for this reaction would be K sp B b C c. Web Since the concentration of the solid Ca OH 2 is a constant it maybe included in the Keq for the reaction and a new constant Ksp the Solubility Product is.
For any equilibrium question and Ksp problems are just a special case of equilibrium its always helpful to start with the equation of the equilibrium. For example the acetic acid here is. Web The first thing to do is identify the values of n and m by writing the dissociation equilibrium for magnesium hydroxide MgOH2s Mg2 aq 2OH aq.
Web Solubility product. Web How to Determine Ksp Given Molar Solubility - YouTube 000 754 How to Determine Ksp Given Molar Solubility 968 views Mar 18 2017 32 Dislike Share Save Prerak. We can calculate the molar solubility using Ksp but we.
The equilibrium for the salt is as follows. Solubility indicates the maximum amount of a substance that can be dissolved in a solvent at a. Let S the solubility saturation concentration of the Ca 2 ion in moles per liter.
Introduction to chemistry - lumen learning. 1 When AgBr dissolves it dissociates like this. Web Relating Solubilities to Solubility Constants.
Solubility data can be used to calculate the Ksp for a given compound. The following steps need to be taken. Determine the K sp of silver bromide given that its molar solubility is 571 x 10 7 moles per liter.
The known K s p values from Table above can be used to calculate the solubility of a given compound by following the. Web Explains how to calculate solubilities from Ksp values. Web Calculating Ksp from Solubility Read Chemistry CK-12 Foundation Calculating Ksp from Solubility Demonstrates calculations used to relate solubility.
The Ksp expression is. The solubility by which we usually mean the molar solubility of a solid is expressed as the concentration of the dissolved solid in a. Then 2S the.
Since all products come from the same reactant they will always be produced in.

The Solubility Product Constant Ksp Ppt Download

How To Calculate Molar Solubility From Ksp Youtube

Solubility Of Agi In 1 0m Nh3 Ksp And Complex Ion Formation Youtube

Worked Example Calculating Solubility From Kₛₚ Equilibrium Ap Chemistry Khan Academy Youtube

The Solubility Product Constant Biochemhelp

The Solubility Of Pbi2 At 25 O C Is 0 7 G L 1 The Solubility Product Of Pbi2 At This Temperature Is Molar Mass Of Pbi2 461 2 G Mol 1

Ksp And Molar Solubility Calculations Video Youtube

Using Ksp To Calculate The Solubility Of A Compound Chemistry Study Com

How To Determine Ksp Given Molar Solubility Youtube
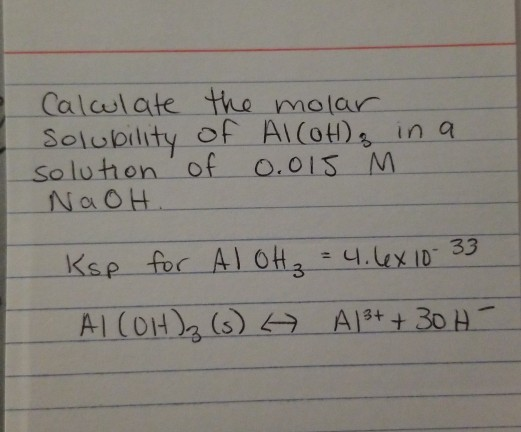
Solved I Calculate Solubility Solution Naoh The Molar Of Chegg Com

Solubility Product Of Agcl Is 2 8xx10 10 At 25 C Calculate Solubility Of The Salt Youtube

How To Find Molar Solubility And Ksp Real Chemistry Youtube

Using Ksp To Find Solubility Youtube
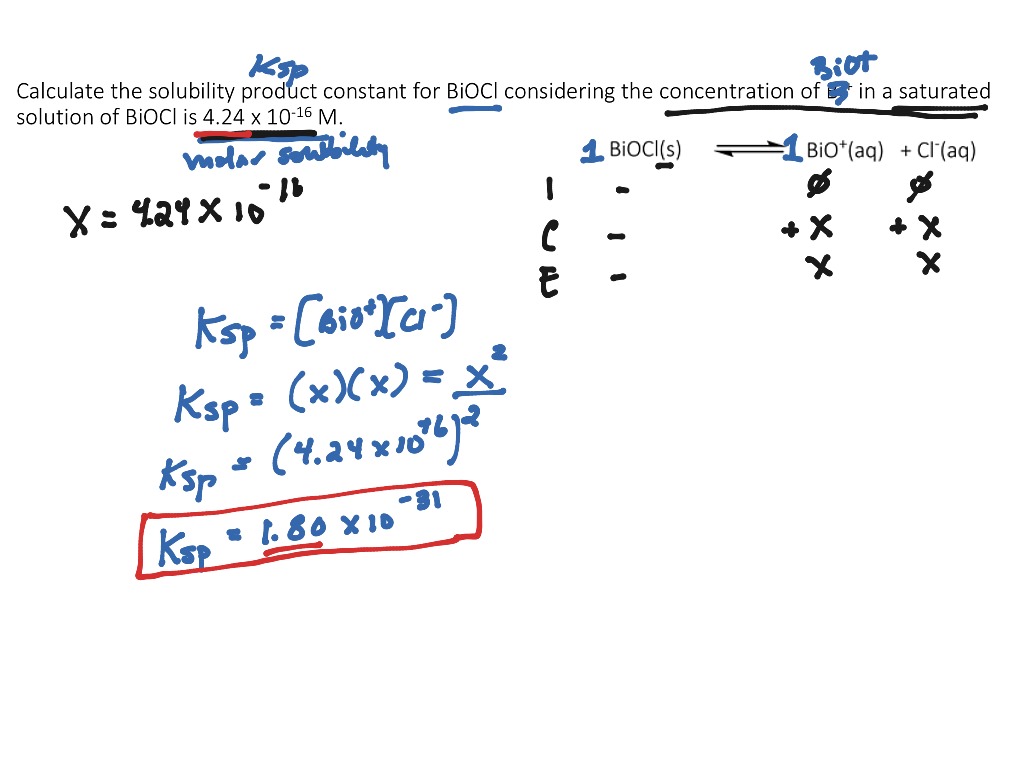
Calculating The Solubility Product Constant Ksp For An Insoluble Salt Science Chemistry Showme

Solubility Equilibria And Temperature Intro Theory Youtube

Worked Example Calculating Solubility From Kₛₚ Equilibrium Ap Chemistry Khan Academy Youtube

Calculate Ksp From Molar Solubility Problem 86 Youtube